Feature
- Compact, lightweight, desktop design
- Integrated weighing, temperature, pH/conductivity
- Magnetic drive, seal-free mixing paddle
- Touchscreen control, easy to operate
- Compatible with LeKrius® mixing bags
- GMP-grade compatible
Application
The Minwind Tabletop Mixing System is applicable to biopharmaceutical R&D and production:
- Intermediate homogenization
- Lab-scale mixing
- Adjuvant processing
- Semi-finished prep
- Viral inactivation
- Pre-filling homogenization
Design Feature
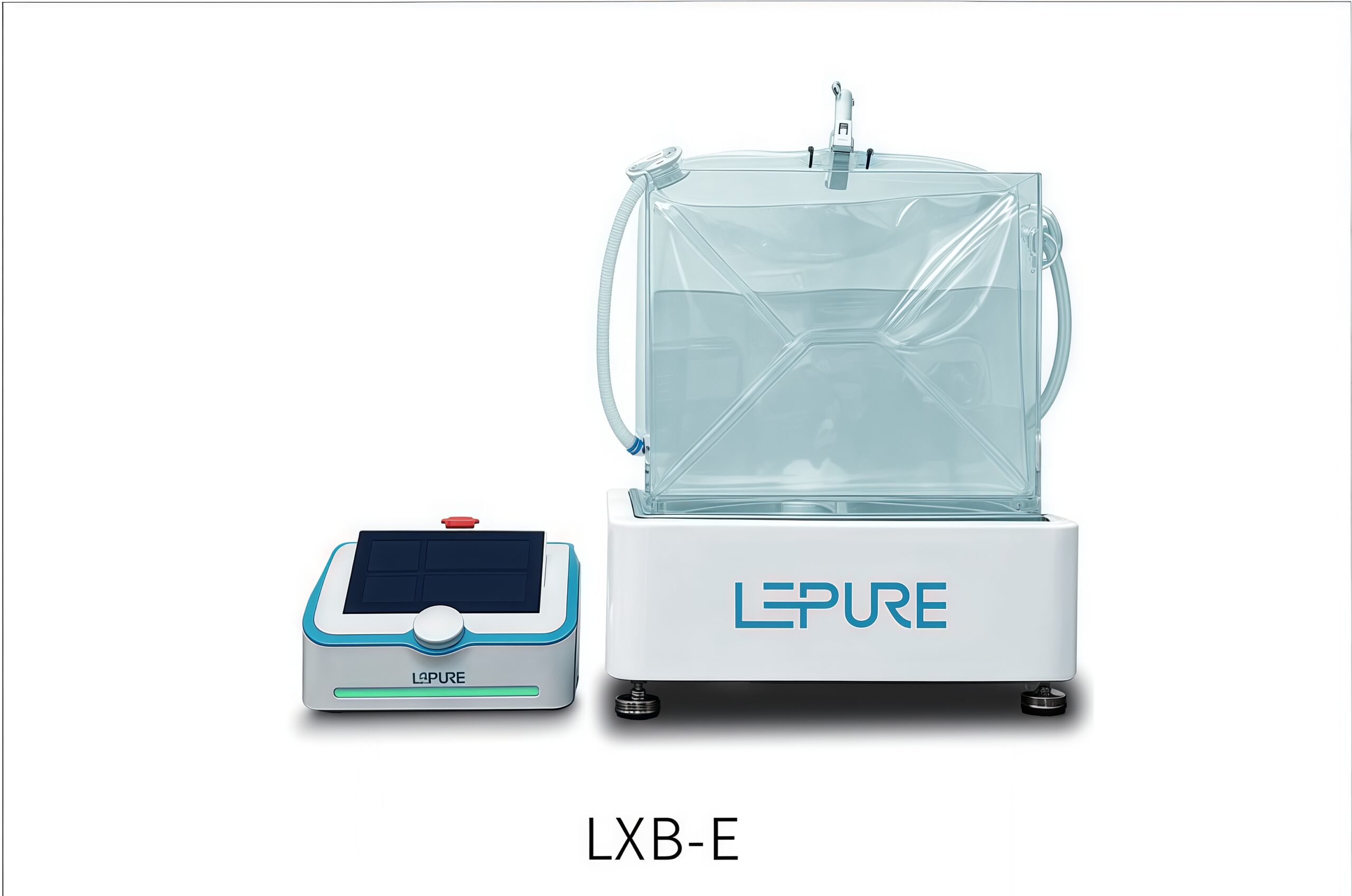
The Minwind Tabletop Mixing System is designed for sterile liquid mixing, supporting homogenization from 500 mL to 30 L and bridging volume gaps in traditional systems. It uses magnetic coupling for contactless power transfer, eliminating shaft seal contamination. Pre-sterilized bags require no cleaning, and the transparent design allows real-time monitoring,ensuring efficient, safe, and sterile mixing for small-volume applications.
Software Control and Functions
- Compact design with small footprint
- Lightweight design enables easy benchtop deployment in lab-scale or controlled environments.
- Magnetic drive, seal-free, contamination-free
- Modular sensor ports pre-configured
- Compatible with 75% ethanol, isopropanol wiping
Functional Modules
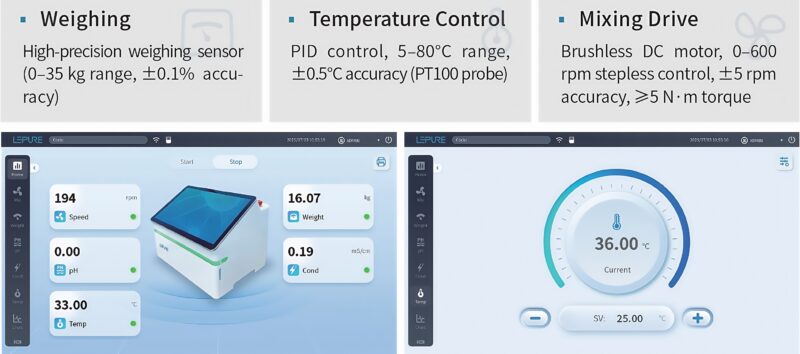
Process Detection Module

Software & Human‒Machine Interface (HMI)
- Proprietary software with fast response
- Bilingual (Chinese-English) support
- Easy type: 7″ display; Power type: 15.6″ HD touchscreen with clear UI
- Layered menus showing mixing speed, temp, pH/conductivity, and weight in real time
- Supports user permissions, audit trail, data logging, and batch reporting
- Ethernet port for SCADA, MES, and centralized system integration
Compliance
- Four-level permission control with 7 automatic logs (audit trail, operation, etc.) and PDF report export
- fully GMP compliant
- Level 2 electronic signature meeting 21 CFR Part 11 data integrity standards
- CE certified, compliant with EU regulations
Compatible Consumables
Independently developed:LeKrius® Film
- Safety
- Robustness
- Sustainability
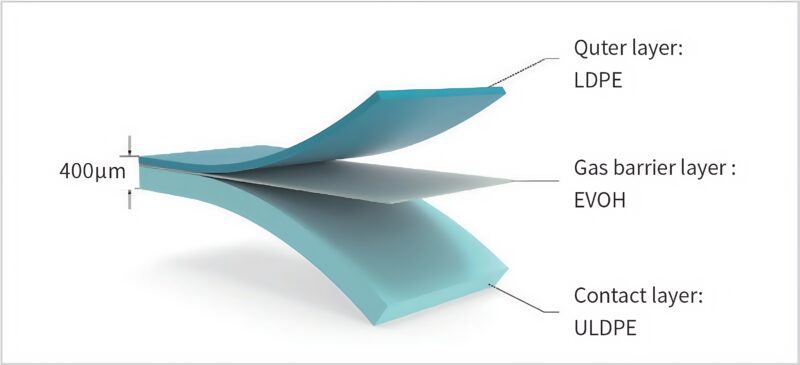
LeKrius®Film is a co-extruded multi-layer film meticulously made of LDPE-EVOH-ULDPE materials.
These materials undergo stringent biocompatibility testing, guaranteeing an exceptionally low level of extractables.
The film has a thickness of 0.4 mm and is sterilized using gamma irradiation at a dose exceeding 25 kGy.
The maximum radiation tolerance is 50 kGy.
Both the raw materials and the manufacturing process of the LeKrius® film are free from TSE/BSE risk.
Advantages
- Comprehensive qualification with rigorous biocompatibility tests
- Great performance of cell cultivation, extremely low level of extractables and leachabels
- Assurance of supply and batch-to-batch consistency
- Low gas transmission, high sealing strength and high resistance to blending and rubbing