Trusted Single-Use Bag Provider with 10+ Years of Innovation
With over a decade of dedicated innovation, LePure Biotech delivers a comprehensive portfolio of high-performance bioprocessing bags tailored for liquid storage, powder handling, drug formulation, and other critical applications. Our solutions are engineered for speed, reliability, cost efficiency, and regulatory compliance.
Driven by a relentless focus on technological advancement, we continuously enhance our manufacturing capabilities to meet the evolving needs of biopharmaceutical production. At the core of our offering is the proprietary LeKrius® Film, a next generation material developed in-house to ensure exceptional stability, low extractables, and outstanding versatility. From upstream cell culture to final fill, LeKrius® empowers breakthrough bioprocessing performance.
Single-Use Bag Product Overview
LePure Biotech offers a wide range of single-use bags with volumes ranging from 5mL to 3000L, all tailored to your specific needs. Backed by in-house expertise, we are committed to delivering top-quality products and exceptional services.
Featured Products

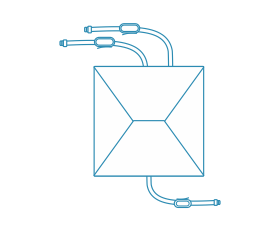
LeKrius® Storage Bags
Leveraging our LeKrius Film, our LeKrius Storage Bags epitomize stability and convenience for cell culture growth. Available in a comprehensive series comprising 2D, 2.5D, and 3D Storage Bags, these cutting-edge solutions cater to volume specifications ranging from 5mL to 2500L, ensuring versatility to meet diverse needs.

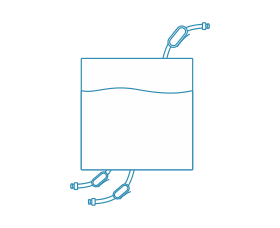
Mixing Bags
Our Mixing Bags offer unparalleled versatility, available as both standard and customizable solutions tailored precisely to your requirements. With sizes ranging from 500mL to 3000L, these bags facilitate seamless scale-up, ensuring stable mixing effects with utmost ease and efficiency.

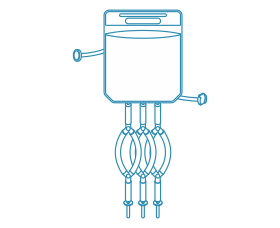
Filling Bags
LePure’s Filling Bags offer flexible, space-saving alternatives to traditional stainless steel tanks. Manufactured with ultra-pure processes, they’re customizable to fit different equipment and processes. Our expert support team ensures seamless integration, saving you time and costs.
Quality Assurance
Drawing upon our team’s extensive industry expertise and continuous awareness, LePure Biotech proudly presents FDA-registered medicinal single-use systems, facilitating swift FDA product registration for our partners.
Moreover, we offer:
- Comprehensive documentation
- Thorough system validation testing
- Adherence to regulatory standards
- Full-process traceability
- Reliable supply guarantees


Supply Security
To ensure uninterrupted business operations, LePure Biotech maintains multiple production bases strategically located across the globe:
- Several production plants in key regions including Shanghai, Tianjin, Suzhou, and the South China Bay Area in China.
- An advanced R&D center situated in Boston, USA.
- A dedicated business office serving the Korean market.