Meet All Your Liquid Storage and Transportation Needs
With over a decade of expertise in single-use technologies, LePure Biotech offers a full range of LeKrius ®️ Liquid Storage Bags, available in 2D and 3D formats, crafted from our proprietary multi-layer coextrusion films. Standard volumes range from 5 mL to 2,500 L, with flexible customization options to meet diverse application needs.
Powered by advanced LeKrius ®️ bioprocess films, our bags deliver ultra-low gas permeability, robust mechanical strength, broad chemical compatibility, and proven biocompatibility to support optimal cell growth. Backed by a stable supply chain and rigorous quality control, LeKrius ®️ ensures consistent performance batch after batch, providing safe, reliable, and convenient storage and transport for your critical liquids.
From 5 mL to 2500 L, Lekrius Storage Bags are all tailored to your specific needs.
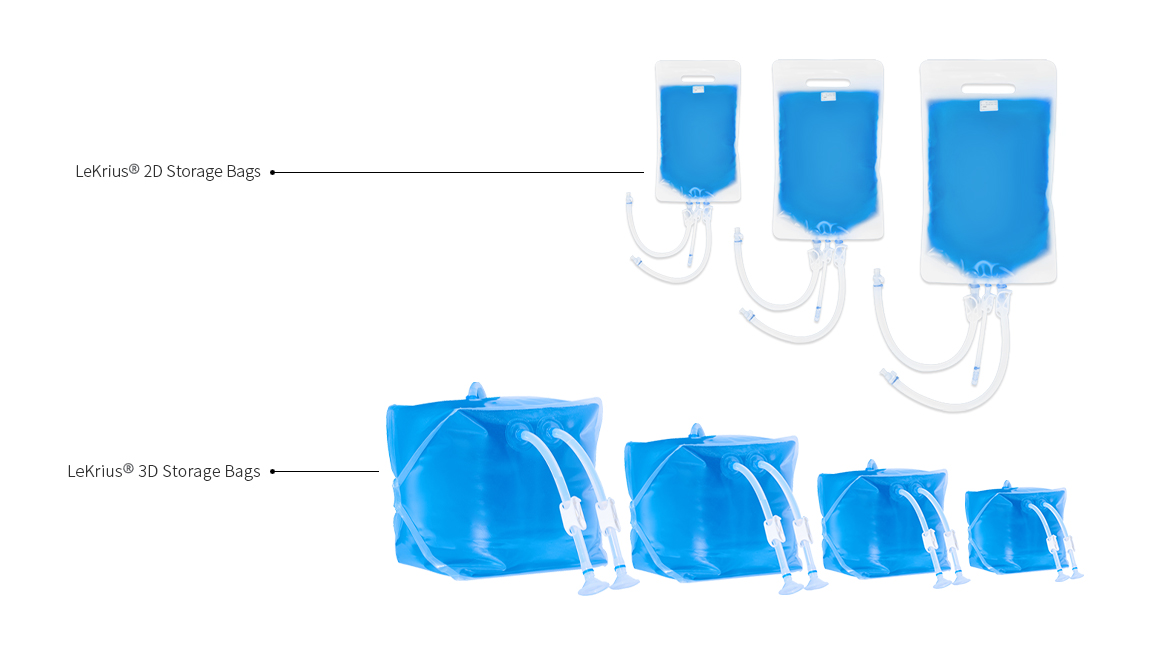
Types and Sizes
2D Storage Bag
 Learn More
Learn More

- Completely upgraded design in 2023
- Wedge-shaped design, combined with a boat-shaped mouth, achieving a solution drainage rate of over 99.9%
- Coupled with the integral molding process, providing a more stable quality of solution transport
- Can be used in conjunction with liquid transport vehicles for storing and transporting small volumes of fluids
3D Storage Bag
 Learn More
Learn More

- Compatible with foldable square boxes and square liquid storage carts
- Supports large-volume fluids (250 L/ 500 L/ 1000 L)
- Customizable capacity for tailored solutions
Case Study
Experiment Purpose
To test the impact of different membrane materials on CHO K1 and CHO DG44 cell growth density and cell viability
Experiment Parameters
- Cell Types: CHO K1 and CHO DG44
- Culture Conditions: 37℃, 3 days
- Area-to-Volume Ratio: 3 cm²/mL
Experiment Results
- Live cell density is comparable to bottle culture, superior to the competitor
- Over the 14-day cell culture process, cell viability is close to perfection

LeKrius®️Film: Advanced Biocompatible Solution
Our LeKrius®️ film, constructed from LDPE-EVOH-ULDPE materials, undergoes rigorous biocompatibility testing. With minimal extractables and a TSE/BSE-free production process, it ensures the reliability and strength of single-use bags with high heat seal strength and robust resistance to bending and abrasion.


Comprehensive Quality Control System for Production Safety
The LeKrius®️ Film membrane is produced in a Class D cleanroom. To ensure safety, we continuously conduct dynamic environmental monitoring during the production of the LeKrius membrane.
Test data indicates that the air environment around the coextrusion membrane equipment, including the quantity of dust particles and settled microorganisms, meets dynamic Class C standards.
Additionally, every step of the process bag manufacturing undergoes real-time monitoring. These measures are implemented to ensure the quality and safety of the product.
Standard Product Parameters:
Please contact sales for customization needs.

| Volume | Inlet Tubing | Outlet Tubing | Sampling Tubing | Product Number |
|---|---|---|---|---|
| 50mL | 1/8"*1/4" L-Flex TPE 30cm+Luer female fitting+male plug | 1/8"*1/4" L-Flex TPE 30cm+Luer male connector+female plug | / | HP0050-1AK |
| 100mL | 1/8″*1/4″ L-Flex 30cm + Luer Female connector + Male luer Cap | 1/8″*1/4″ L-Flex 30cm+Luer Male connector+Female luer Cap | / | HP0100-1AK |
| 250mL | 1/4"*3/8" L-Flex TPE 30cm+MPC female connector + male cap | 1/4"*3/8" L-Flex TPE 30cm+MPC male connector+female cap | / | HP0250-3AK |
| 500mL | 1/4"*3/8" L-Flex TPE 30cm+MPC female connector + male cap | 1/4"*3/8" L-Flex TPE 30cm+MPC male connector+female cap | / | HP0500-3AK |
| 1L | 1/4"*3/8" L-Flex TPE 50cm+MPC female connector+male cap | 1/4"*3/8" L-Flex TPE 50cm+MPC male connector+female cap | 1/4"*3/8"Lepure Silicone Tubing 20cm+ Female Luer Thread Barb + Needleless Sampler + Needleless Sampler cap | HP001-4AK |
| 2L | 1/4"*3/8" L-Flex TPE 50cm+MPC female connector+male cap | 1/4"*3/8" L-Flex TPE 50cm+MPC male connector+female cap | 1/4"*3/8"Lepure Silicone Tubing 20cm+ Female Luer Thread Barb + Needleless Sampler + Needleless Sampler cap | HP002-4AK |
| 3L | 1/4"*3/8" L-Flex TPE 50cm+MPC female connector+male cap | 1/4"*3/8" L-Flex TPE 50cm+MPC male connector+female cap | 1/4"*3/8"Lepure Silicone Tubing 20cm+ Female Luer Thread Barb + Needleless Sampler + Needleless Sampler cap | HP003-4AK |
| 5L | 3/8"*5/8" L-Flex TPE 50cm+MPC female connector+male cap | 3/8″*5/8″ L-Flex TPE 50cm+MPC male connector+Female cap | 1/4"*3/8" Lepure Silicone Tubing 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | HP005-5AK |
| 10L | 3/8"*5/8" L-Flex TPE 50cm+MPC female connector+male cap | 3/8″*5/8″ L-Flex TPE 50cm+MPC male connector+Female cap | 1/4"*3/8" Lepure Silicone Tubing 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | HP010-5AK |
| 20L | 3/8"*5/8" L-Flex TPE 50cm+MPC female connector+male cap | 3/8″*5/8″ L-Flex TPE 50cm+MPC male connector+Female cap | 1/4"*3/8" Lepure Silicone Tubing 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | HP020-5AK |
| 30L | 3/8"*5/8" L-Flex TPE 50cm+MPC female connector+male cap | 3/8″*5/8″ L-Flex TPE 50cm+MPC male connector+Female cap | 1/4"*3/8" Lepure Silicone Tubing 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | HP030-5AK |
| 50L | 3/8"*5/8" L-Flex TPE 50cm+MPC female connector+male cap | 3/8″*5/8″ L-Flex TPE 50cm+MPC male connector+Female cap | 1/4"*3/8" Lepure Silicone Tubing 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | HP050-5AK |
| 1L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP001-5DK |
| 2L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP002-5DK |
| 5L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP005-5DK |
| 10L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP010-5DK |
| 20L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP020-5DK |
| 50L | 3/8"*5/8" L-Flex TPE 60cm+-Sealed end | 3/8"*5/8"L-Flex TPE 60cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP050-5DK |
| 1L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP001-5BK |
| 2L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP002-5BK |
| 5L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP005-5BK |
| 10L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP010-5BK |
| 20L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP020-5BK |
| 50L | 3/8"*5/8"L-Flex TPE 50cm+-filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" L-Flex TPE 50cm+-Sealed end | 1/4"*3/8" L-Flex TPE 20cm+Female Luer Thread Barb+Needle-less Sampler+Needle-less Sampler cap | HP050-5BK |
| 1L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP001-5EK |
| 2L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP002-5EK |
| 5L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP005-5EK |
| 10L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP010-5EK |
| 20L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP020-5EK |
| 50L | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing50cm+MPC male connector+female cap | 1/4"*3/8" Lepure Silicone Tubing15cm+Luer female fitting+male plug | HP050-5EK |
| 1L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP001-5CK |
| 2L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP002-5CK |
| 5L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP005-5CK |
| 10L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP010-5CK |
| 20L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP020-5CK |
| 50L | 3/8"*5/8" Lepure Silicone Tubing50cm(Add and disconnect loops)+filter(P1S5DS5THM)+3/8"*5/8" Lepure Silicone Tubing30cm+MPC female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing 50cm+TC25+Tri-Clamp | 1/4"*3/8" Lepure Silicone Tubing20cm+Luer female fitting+male plug | HP050-5CK |
| 57L | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT057-7K |
| 113L | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT113-7K |
| 208L | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 3/8"*5/8" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT208-7K |
| 303L | 1/2"*3/4" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/2"*3/4" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT303-7K |
| 378L | 1/2"*3/4" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/2"*3/4" Lepure Silicone Tubing150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT378-7K |
| 57L | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT057-8K |
| 113L | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT113-8K |
| 208L | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 3/8"*5/8" L-Flex TPE 150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT208-8K |
| 303L | 1/2"*3/4" L-Flex TPE 150cm+MPX male connector+female cap | 1/2"*3/4" L-Flex TPE 150cm+MPX male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT303-8K |
| 378L | 1/2"*3/4" L-Flex TPE 150cm+MPX male connector+female cap | 1/2"*3/4" L-Flex TPE 150cm+MPX male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FT378-8K |

| Volume | Inlet Tubing | Outlet Tubing | Sampling Tubing | Product Number |
|---|---|---|---|---|
| 250L | 3/8"*5/8" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 3/8"*5/8" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-7K |
| 500L | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD500-7K |
| 1000L | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD1000-7K |
| 250L | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-8K |
| 500L | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD500-8K |
| 1000L | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 3/8"*5/8" Lepure Silicone Tubing150cm+MPC male connector+female cap | 1/8"*1/4" Lepure Silicone Tubing30cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD1000-8K |
| 100L | 1/2"*3/4" L-Flex TPE 60cm+Sealed end | 1/2"*3/4" Lepure Silicone Tubing50cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD100-11K |
| 200L | 1/2"*3/4" L-Flex TPE 60cm+Sealed end | 1/2"*3/4" Lepure Silicone Tubing50cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-11K |
| 250L | 1/2"*3/4" L-Flex TPE 60cm+Sealed end | 1/2"*3/4" Lepure Silicone Tubing50cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-11K |
| 500L | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD500-11K |
| 1000L | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4"L-Flex TPE 60cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD1000-11K |
| 100L | 1/2"*3/4"L-Flex TPE 100cm+filter(P1S5D05THM)+1/2"*3/4" Lepure Silicone Tubing50cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4" L-Flex TPE 50cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD100-9K |
| 200L | 1/2"*3/4"L-Flex TPE 100cm+filter(P1S5D05THM)+1/2"*3/4" Lepure Silicone Tubing50cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4" L-Flex TPE 50cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-9K |
| 250L | 1/2"*3/4"L-Flex TPE 100cm+filter(P1S5D05THM)+1/2"*3/4" Lepure Silicone Tubing50cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4" L-Flex TPE 50cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD250-9K |
| 500L | 1/2"*3/4" L-Flex TPE 100cm+filter(P1S5D20TBM)+1/2"*3/4" Lepure Silicone Tubing50cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4" L-Flex TPE 50cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD500-9K |
| 1000L | 1/2"*3/4" L-Flex TPE 100cm+filter(P1S5D20TBM)+1/2"*3/4" Lepure Silicone Tubing50cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing100cm+1/2"*3/4" L-Flex TPE 50cm+Sealed end | 1/4"*3/8" Lepure Silicone Tubing6cm+1/8"*1/4" L-Flex TPE 20cm+Female Luer Thread Barb+Needleless Sampler+Needleless Sampler cap | FD1000-9K |
| 100L | 1/2"*3/4" Lepure Silicone Tubing 150cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing15cm+Luer female fitting+male plug | FD100-12K |
| 200L | 1/2"*3/4" Lepure Silicone Tubing 150cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing15cm+Luer female fitting+male plug | FD200-12K |
| 250L | 1/2"*3/4" Lepure Silicone Tubing 150cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing15cm+Luer female fitting+male plug | FD250-12K |
| 500L | 1/2"*3/4" Lepure Silicone Tubing 150cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing15cm+Luer female fitting+male plug | FD500-12K |
| 1000L | 1/2"*3/4" Lepure Silicone Tubing 150cm+MPX female connector+male cap | 1/2"*3/4" Lepure Silicone Tubing 150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing15cm+Luer female fitting+male plug | FD1000-12K |
| 100L | 1/2"*3/4" Lepure Silicone Tubing 50cm(Add and disconnect loops)+filter(P1S5D04THM)+1/2"*3/4" Lepure Silicone Tubing100cm+MPX female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing 20cm+Luer female fitting+male plug | FD100-10K |
| 200L | 1/2"*3/4" Lepure Silicone Tubing 50cm(Add and disconnect loops)+filter(P1S5D04THM)+1/2"*3/4" Lepure Silicone Tubing100cm+MPX female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing 20cm+Luer female fitting+male plug | FD250-10K |
| 250L | 1/2"*3/4" Lepure Silicone Tubing 50cm(Add and disconnect loops)+filter(P1S5D04THM)+1/2"*3/4" Lepure Silicone Tubing100cm+MPX female connector+male cap | 3/8"*5/8" Lepure Silicone Tubing150cm+TC25+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing 20cm+Luer female fitting+male plug | FD250-10K |
| 500L | 1/2"*3/4" Lepure Silicone Tubing 50cm(Add and disconnect loops)+filter(P1S5D10THM)+1/2"*3/4" Lepure Silicone Tubing100cm+MPX female connector+male cap | 1/2"*3/4"Lepure Silicone Tubing150cm+ TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing 20cm+Luer female fitting+male plug | FD500-10K |
| 1000L | 1/2"*3/4" Lepure Silicone Tubing 50cm(Add and disconnect loops)+filter(P1S5D10THM)+1/2"*3/4" Lepure Silicone Tubing100cm+MPX female connector+male cap | 1/2"*3/4"Lepure Silicone Tubing150cm+ TC50+Tri-Clamp | 1/8"*1/4" Lepure Silicone Tubing 20cm+Luer female fitting+male plug | FD1000-10K |

